Rutherford's gold foil experiment was a major milestone in the determination of the size and proportions of the different constituents of the atom. It was successful in determining that the majority of the mass of an atom lies within the nucleus, just as the majority of the mass of a planetary system lies within the stellar system of that planetary system.
Rutherford measured the amount and frequency of deflection of alpha particles as they were shot through a very thin gold foil. Ruthford, How I did the experiment This helped to determine that the nucleus of the atom is contained to a very small portion of the atom, at it's center. The deflection of the alpha particles was incorrectly reasoned to be due to the like charges of the alpha particle and the nucleus of the gold atoms. This incorrect reasoning was no fault of Rutherford, however, as by that time in history, the concept of electric charges within the atom had already been incorrectly established as the reason for attraction and repulsion of the atomic constituents. So, it was obvious that the alpha particles were being deflected by the nucleus, but the reason for the deflection was misconstrued, no fault of Rutherford's.
In the AAM, there is no concept of electric charge. All matter is attracted to other matter, by the same force which is responsible for gravity. Thus, the alpha particles which pass through the gold foil and pass close enough to the nucleus of a gold atom are attracted to the nucleus, which causes the alpha particle trajectory to bend towards the nucleus, not away from it, as shown in figure 2 below.
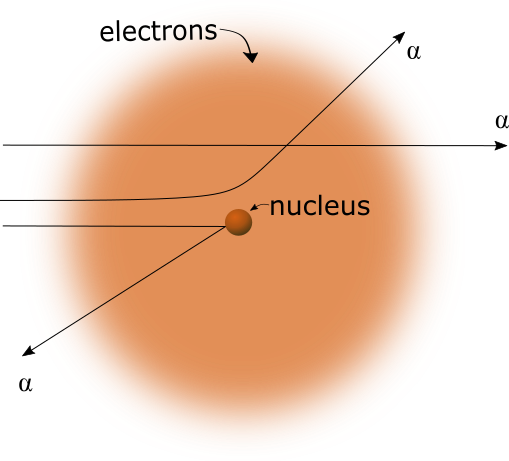
Figure 1
Conventional explanation for deflection of alpha particles. Alpha particles are repelled by the nucleus.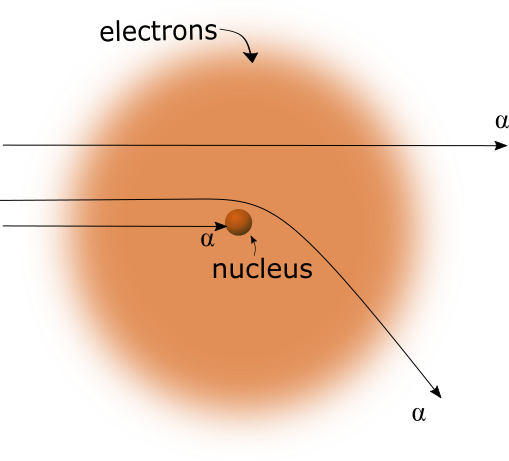
Figure 2
Correct explanation for deflection of alpha particles. Alpha particles are attracted to the nucleus.The experimental results of either case above will be very much the same. There would be small differences, however, which may be able to determine which case is actually correct. In the conventional explanation, as shown in Figure 1 above, it's possible for an alpha particle to be deflected 180%, and return strait back to the source in which it came. In the second case, this would not be possible, since the alpha particle would collide with the gold nucleus.
Modification of the gold foil experiment may also help to determine which case is valid. If the alpha particles were to be show slowly in the gold foil, the patterns of deflections may be calculated to be different for each case, since the repulsive force in the first case would cause a different amount of deflection than the attractive forces in the second case. So, this experiment would be very good to revive, with this new concept in mind, and with keeping an open mind to the possibility that it is an attractive force causing the deflections of the alpha particles.
For the discussion on why the alpha particles are attracted to the nucleus, please see Gravity and Electric Charge.
Submit Comment
test discussion